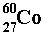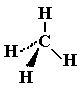Molecule
A MOLECULE is a larger particle formed by the chemical combination of two or more atoms. The molecule may be an element e.g. hydrogen H2 (two atoms combined) or a compound (more examples below) e.g. carbon dioxide CO2 (three atoms combined) and in each case the atoms are held together by chemical bonds. (Detailed GCSE bonding notes and examples) You can represent molecule in various styles of diagram. For example, you can colour and size code the atoms of different elements, so in the molecule pictured on the left, you can tell there are five types of atom (elements) and six atoms in total in the molecule.
ELEMENT and symbols
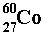
- An ELEMENT is a pure substance made up of only one type of atom*, 92 in the Periodic Table (detailed notes) naturally occur from hydrogen H to uranium U.
- Note that each element has symbol which is a single capital letter like H or U or a capital letter + small letter e.g. cobalt Co, calcium Ca or sodium Na.
- Each element has its own unique set of properties but the Periodic Table is a means of grouping similar elements together. They may exist as atoms like the Noble Gases e.g. helium He or as molecules e.g. hydrogen H2 or sulphur S8.(more examples applied to equations)
- * At a higher level of thinking, all the atoms of the same element, have the same atomic or proton number. This number determines how many electrons the atom has, and so ultimately its chemistry. Any atom with 27 protons and electrons is cobalt! The diagram 60-Co-27 uses advanced notation - all explained on the atomic structure page.
- See also picture diagrams of elements/compounds and mixtures.
- Elements are broadly divided by physical and chemical character into metals and non-metals. A few elements display characteristics of metallic and non-metallic elements and are referred to as semi-metals or metalloids. Elements can be more highly characterised and organised in the form of the Periodic Table. All of these points are discussed on the GCSE Periodic Table notes page.
CHEMICAL BOND
- The varieties of chemical substances around you are all due to different combinations of atoms. Atoms combine or 'connect' together by means of chemical bonds. KS3 students do NOT need to know about chemical bonding, but the result is that millions of different substances (molecules/compounds) can exist because of the huge variety of atom combinations possible.
- Detailed GCSE bonding notes and examples for ionic, covalent and metallic bonds.
FORMULA and MOLECULE
- The chemical composition of a pure substance can be represented using the element symbols, and where necessary, subscripted numbers. A formula represents the relative numbers of atoms of each element in a substance.
- Note that the formula of a particular pure substance does NOT change just because it becomes part of a mixture. If it retains its own chemical identity in a mixture, i.e. it does not change chemically, then it must have the same formula.
- You need to be able to read a formula e.g. like the one below.
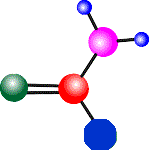
- The first 'picture' is an example of a displayed formula, in which every individual atom is shown and how it is bonded ('connected') with other atoms in the molecule. All the dashes represent the covalent bonds between the atoms in the molecule.
- From this diagram you can tell there are four different elements in the molecules and the number of atoms of each element ...
- ... there are 4 carbon atoms (C), 8 hydrogen atoms (H), 1 bromine atom (Br) and 1 chlorine atom (Cl) and because there are at least 2 different elements chemically combined in the molecule or formula, this also tells you it is a compound (see below for more examples).
- A summary of all the atoms in the individual molecule is called the molecular formula, shown on the right.
- There are more examples in the next section which discusses the word compound further.
- Also, look at an example where they are used in chemical equations - burning methane.
- Note: A displayed formula is sometimes called a full structural formula or graphic formula.
More on formulae and COMPOUNDS
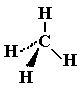
· A COMPOUND is a pure substance formed by chemically combining at least two different elements by ionic or covalent bonding.
· Compounds can be represented by a FORMULA, which represents the whole number (integer) ratio of the atoms in a formula, and for molecules, a summary of all the atoms in one molecule. Examples:- sodium chloride NaCl, ionic compound, 2 elements, 1 of sodium and 1 of chlorine).
- methane CH4, covalent compound molecule, has 2 elements in it, 4 atoms of carbon and 1 atom of hydrogen*. The lines in the diagram of methane
- ethane C2H6, two atoms of carbon combined with 6 atoms of hydrogen and note that two elements can form two different compounds because of different atom ratios.
- glucose C6H12O6 (covalent compound molecule, 3 elements in it, 6 atoms of carbon, 12 of hydrogen and 6 of oxygen).
- carbon monoxide CO (1C + 1O atoms), carbon dioxide CO2 (1C + 2O atoms), note again that two elements can form two different compounds because of different atom ratios.
NOTE
There must be at least two different types of atom (elements) in a compound.
The number of atoms of each element is shown as a subscript number in the formula ...
... except * the 1 is never written in the formula, no number means 1.
· Compounds have a fixed composition and therefore a fixed ratio of atoms represented by a fixed formula, however the compound is made or formed.
· In a compound the elements are not easily separated by physical means, and quite often not easily by chemical means either.
· The compound has properties quite different from the elements it is formed from. For example the two elements soft silvery reactive sodium + reactive green gas chlorine ==> colourless, and not very reactive crystals of the compound sodium chloride.
· The FORMULA of a compound summarizes the 'whole number' atomic ratio of what it is made up of e.g.methane CH4 is composed of 1 carbon atom combined with 4 hydrogen atoms. Glucose has 6 carbon : 12 hydrogen : 6 oxygen atoms, sodium chloride is 1 sodium : 1 chlorine atom.
· Sometimes, a compound (usually ionic), is partly made up of two or more identical groups of atoms. To show this more accurately ( ) are used e.g.
- Calcium hydroxide is Ca(OH)2 which makes more sense than CaO2H2 because the OH group of atoms is called hydroxide and exists in its own right in the compound.Make sure you understand the use of (brackets). The subscripted 2 after the brackets doubles everything in the brackets BUT nothing else in the formula.
- Similarly, aluminium sulphate has the formula. Al2(SO4)3 rather than Al2S3O12, because it consists of two aluminium ions Al3+ and three sulphate ions SO42-. The sulphate ion is effectively a molecule that carries an overall surplus electrical charge - a 'molecular ion' if you like.
· The word formula or molecule can also apply to element. e.g. hydrogen molecule H2, oxygen molecule O2, ozone molecule O3 (2nd unstable form of oxygen), phosphorus molecule P4, sulphur molecule S8, have 2, 2, 3, 4 and 8 atoms in their molecules. Elements like helium He are referred to as 'monatomic' because they exist as single uncombined atoms.
- Incidentally, at GCSE level, and mainly at Advanced level too, phosphorus and sulfur are written as P and S respectively. However, in equations
· There are more examples and comments in equation section.
· Calculations involving empirical formula and molecular formula are dealt with in sections 5. and 8. on the calculations page.
· See also picture diagrams of elements/compounds and mixtures.
MIXTURE
- A MIXTURE is a material made up of at least two substances which may be elements or compounds. They are usually easily separated by physical means e.g. filtration, distillation, chromatography etc. Examples: air, soil, solutions. Separation methods are needed to separate useful materials and purify them.
- See also picture diagrams of elements/compounds and mixtures.
PURE
- PURE means that only one substance is present in the material and can be a pure element or compound.
- A simple physical test for purity, and properties that can help identify a substance, is to measure the boiling point or melting point. Every pure substance melts and boils at a fixed temperature (though boiling point depends on the ambient air pressure).
- If a liquid is pure it should boil at a constant temperature called the boiling point e.g. water boils at 100oC. Unfortunately, up on a very high mountain, at a lower air pressure, water boils at a constant, but lower temperature and it is difficult to make a good brew of tea!
- An impure liquid will boil at a higher temperature if it contains a dissolved solid impurity e.g. seawater, containing dissolved salts, boils at over 100oC.
- An impure liquid can initially boil at a lower than the expected temperature, if it contains a lower boiling point liquid impurity. The boiling then takes place over a range of temperatures. For example, in the distillation of an alcohol-water mixture from a fermented yeast-sugar solution mixture, it boils away within a range starting at about 79oC (boiling point of alcohol) and the last drops distil over at 100oC (boiling point of pure water).
- If a solid is pure, it melts sharply at its fixed melting point.
- An impure solid melts below its expected melting point and the more impure, the wider the temperature melting range.
- e.g. a water and salt mixture melts below 0oC and butter, a mixture of fats, gradually melts more as the temperature rises on a hot summer's day.
IMPURE
- IMPURE usually means a mixture of mainly one substance plus one or more other substances physically mixed in.
- Some examples are mentioned above, in the discussion on the effect of impurities on the melting/boiling points of pure substances.
- The % purity of a compound is important, particularly in drug manufacture. Any impurities present are less cost-effective to the consumer and they may be harmful substances.
PURIFICATION
- PURIFICATION: Materials are purified by various separation techniques.
- The idea is to separate the desired material from unwanted material or impurities.
- Detailed examples of methods-examples of separating mixtures are described in later sections on this page.
- but they include:
- Filtration to separate a solid from a liquid. You may want the solid or the liquid or both!
- Simple distillation to separate a pure liquid from dissolved solid impurities which have a very high boiling point.
- Fractional distillation to separate liquids with a range of different boiling points, especially if relatively close together.
- Evaporation to remove a solvent to leave a solid behind.
- Crystallization to get a pure solid out of a solvent solution of it.
- Chromatography can be used on a larger scale than spots' to separate out pure samples from a mixture.
- Methods of collecting gases are on a separate web page.
PHYSICAL CHANGES - no new substance formed

These are changes which do not lead to new substances being formed. Only the physical state of the material changes. The substance retains exactly the same chemical composition. Examples ...
Melting, solid to liquid, easily reversed by cooling e.g. ice and liquid water are still the same H2O molecules.
Dissolving, e.g. solid mixes completely with a liquid to form a solution, easily reversed by evaporating the liquid e.g. dissolving salt in water, on evaporation the original salt is regained.
So freezing, evaporating, boiling, condensing are all physical changes.
Separating a physical mixture e.g. chromatography, e.g. a colored dye solution is easily separated on paper using a solvent, they can all be re-dissolved and mixed to form the original dye.
So distillation, filtering are also physical changes.
CHEMICAL CHANGES - REACTIONS - reactants and products

Heating iron and sulphur is classic chemistry experiment to illustrate what is meant by CHEMICAL CHANGE and you can adapt the general conclusions described at the end of this section to any chemical reaction.
A mixture of silvery grey iron filings and yellow sulphur powder is made.
The iron can be plucked out with a magnet i.e. an easily achieved physical separation because the iron and sulphur are not chemically combined yet!
They are still the same iron and sulphur.
However, on heating the mixture, it eventually glows red on its own and a dark grey solid called iron sulphide is formed. Both observations indicate a chemical change is happening i.e. a new substance is being formed.
We no longer have iron or sulphur BUT a new compound with different physical properties (e.g. colour) and chemical properties (unlike iron which forms hydrogen with acids, iron sulphide forms toxic nasty smelling hydrogen sulphide!).
iron + sulphur (sulfur)==> iron sulphide (iron sulfide) or in symbols: Fe + S ==> FeS
AND it is no longer possible to separate the iron from the sulphur using a magnet!
Further proof of a new substance formed: The original reactant iron, and the iron sulphide product, can be shown to be different substances by their reactions with dilute acid.
Iron forms a pale green solution of the salt iron sulphate with dilute sulphuric acid and evolves odourless hydrogen gas which gives a squeaky pop with a lit splint. The word and symbol equations are as follows ...
iron + sulphuric acid ==> iron sulphate + hydrogen
Fe + H2SO4 ==> FeSO4 + H2
Iron sulphide also fizzes and dissolves in dilute sulphuric acid to form iron sulphate BUT produces the 'rotten eggs' smelly gas hydrogen sulphide which gives a black colour with lead ethanoate paper (old name lead acetate).
iron sulphide + sulphuric acid ==> iron sulphate + hydrogen sulphide
FeS + H2SO4 ==> FeSO4 + H2S
This is NOT to be done by the student, hydrogen sulphide gas is highly poisonous.
If hydrochloric acid is used, the same two colourless gases are produced but the salt formed would be iron chloride.
So signs that a chemical reaction has happened include:
change in appearance e.g. change in colour or texture.
temperature changes because an energy change has taken place,
change in mass e.g.
some solids when burned in air gain mass in forming the oxide e.g. magnesium forms magnesium oxide.
some solids lose mass when heated, e.g. carbonates lose carbon dioxide in thermal decomposition.
and change in the chemical properties of the products compared to the original reactants.
Therefore a chemical change is one in which a new substance is formed, by a process which is not easily reversed and usually accompanied by an energy (temperature) change.
This is summarised as reactants ==> products as expressed in chemical equations in words or symbols.
Apart from experiments and preparations in the laboratory, plenty of chemical changes occur in the home. For a start, you are an extremely complex chemical structure with lots of reactions going on in your body all the time, but others in the home include ...
Cooking involves both physical and chemical changes, e.g. meat and potato change in both taste and texture and breakdown chemically to some extent, baking powder breaks down to release carbon dioxide gas which gives the 'rising action' in the production of cakes etc..
Acidic reagents dissolve limescale in the toilet.
Candles burning at birthdays and Christmas and gas fire also involve combustion of hydrocarbons like methane.
More advanced ideas [see GCSE notes on atomic structure and chemical bonding]: Atoms are held together in molecules or compounds by electrical forces of attraction between the positive nucleus and the outer negative electrons. Therefore, Atoms, ions or molecules react with each other to become electronically more stable. When chemical reactions occur chemical bonds are broken in the reactants and new bonds made in the formation of the products.